| Home Page | Embryology | Anatomy | Innervation | Physiology | Clinic Case |
The urinary bladder and pelvic urethra operate as a functional unit to store and void urine. The unit consists of three components arranged in series:
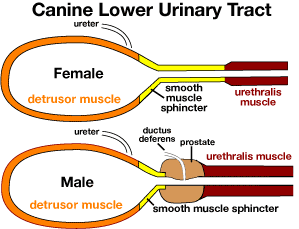 detrusor muscle, acts to expel urine following parasympathetic activation via pelvic nerve innervation;
detrusor muscle, acts to expel urine following parasympathetic activation via pelvic nerve innervation;
smooth muscle sphincter, provides involuntary tonic resistance when activated by sympathetic innervation via hypogastric nerves (sympathetic innervation also inhibits detrusor activity);
striated urethralis muscle, innervated by the pudendal nerve, opposes sudden increases in bladder pressure and is employed for voluntary continence.
The three components are synergistically controlled by the central nervous system. Normal micturition is a voluntary act in which detrusor and sphincter musculature are coordinated to produce complete bladder emptying at appropriate times. (Complete emptying is not a goal when urination is emotionally motivated, e.g., to mark territory.) In neonatal kittens, micturition is reflexly triggered by licking the perineum.
Urine enters the renal pelvis continuously but exits the ureter periodically. Urine accumulation stretches smooth muscle pacemaker cells in the wall of the renal pelvis, triggering them to fire action potentials that lead to increased intracellular [Ca2+] which spreads among myocytes via gap junctions. This results in a peristaltic wave that propels a urine bolus into the bladder with sufficient force to open the terminal intramural ureter, normally held closed by intravesical pressure and wall tension to preclude urine reflux into the ureter.
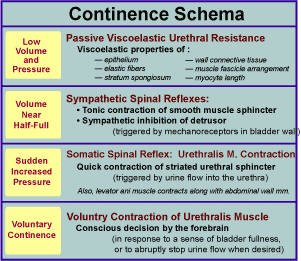 Urinary continence, the ability to store urine without leakage, requires that outlet resistance exceed intravesical pressure. Initially, when bladder volume and pressure are low, passive viscoelastic resistance offered by the urethral wall provides adequate resistance to maintain closure. In quadrupeds, passive resistance is augmented when urine weight pulls the bladder cranially into the abdomen away from the urethra.
Urinary continence, the ability to store urine without leakage, requires that outlet resistance exceed intravesical pressure. Initially, when bladder volume and pressure are low, passive viscoelastic resistance offered by the urethral wall provides adequate resistance to maintain closure. In quadrupeds, passive resistance is augmented when urine weight pulls the bladder cranially into the abdomen away from the urethra.
As bladder volume approaches half-full, continued continence requires that spinal sympathetic reflexes contract the smooth muscle sphincter and inhibit spontaneous contractions of the detrusor. These reflexes involve bladder afferent axons that run in the pelvic nerve to sacral spinal segments and efferent axons that come from lumbar spinal segments and travel though hypogastric nerves to reach the bladder and urethra.
To halt urine leakage during abrupt increases in intravesical pressure, the striated urethralis muscle contracts as a spinal reflex is triggered by urine flow into the urethra. Afferent and efferent axons run through the pudendal nerve. The urethralis muscle is also used for voluntary continence. (In males, the muscle reflexly contracts during ejaculation.)
Bladder volume expansion increases wall tension and mechanoreceptor activity. Afferent axons travel through the pelvic nerve to the sacral spinal cord. They synapse on interneurons and projection neurons. The interneurons generate continence-related spinal reflexes. The projection neurons relay mechanoreceptor status to the brain.
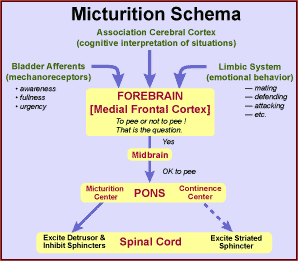 In the forebrain, a sense of bladder fullness or urgency is detected along with cognition of surroundings and consideration of emotional status related to urination. These perceptions are factored into a decision either to inhibit or to initiate micturition. Instructions are sent through the midbrain to the pons.
In the forebrain, a sense of bladder fullness or urgency is detected along with cognition of surroundings and consideration of emotional status related to urination. These perceptions are factored into a decision either to inhibit or to initiate micturition. Instructions are sent through the midbrain to the pons.
The pons switches from continence to micturition by deactivating a pontine continence center and activating a pontine micturition center. The continence center drives the striated urethral sphincter. Descending tracts from the pontine micturition center inhibit spinal neurons to smooth and striated urethral sphincters and excite parasympathetic preganglionic neurons to the detrusor muscle.
Following voluntary relaxation of the striated sphincter, detrusor contraction boosts intravesical pressure and pulls open the bladder neck, forcing urine into the relaxed bladder neck and urethra. Brain facilitation sustains detrusor contraction (even as afferent activity from bladder wall tension receptors declines) until the bladder is empty.
Note: Spinal lesions that damage descending tracts from the pons impair normal micturition by diminishing sustained detrusor contraction and by producing detrusor-sphincter dyssynergy (failure to inhibit sphincter spinal reflexes during detrusor contraction). Dyssynergy impedes attempts to manually empty the bladder in paraplegic patients and chronically results in detrusor hypertrophy and predisposition to cystitis due to retained urine.
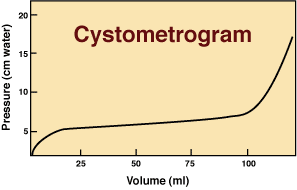 During filling, the normal urinary bladder exhibits high compliance, i.e., the capacity to undergo large volume increases with only minor elevations in intravesical pressure over a wide range of filling capacity. Bladder compliance is a manifestation of the viscoelastic properties of the bladder wall. As the bladder fills, the bladder wall itself is reshaped because incipient tension rearranges muscle fascicles and elongates individual myocytes. Instead of wall tension increasing, it is dissipated in the process of stretching smooth muscle cells and displacing muscle fascicles. Eventually bladder compliance approaches a limit and then, with additional filling, wall tension builds and intravesical pressure consequently rises rapidly.
The clinical cystometrogram (pressure/volume plot) is a manifestation of bladder compliance and its limit.
During filling, the normal urinary bladder exhibits high compliance, i.e., the capacity to undergo large volume increases with only minor elevations in intravesical pressure over a wide range of filling capacity. Bladder compliance is a manifestation of the viscoelastic properties of the bladder wall. As the bladder fills, the bladder wall itself is reshaped because incipient tension rearranges muscle fascicles and elongates individual myocytes. Instead of wall tension increasing, it is dissipated in the process of stretching smooth muscle cells and displacing muscle fascicles. Eventually bladder compliance approaches a limit and then, with additional filling, wall tension builds and intravesical pressure consequently rises rapidly.
The clinical cystometrogram (pressure/volume plot) is a manifestation of bladder compliance and its limit.
During voiding, the detrusor must generate sufficient lumen pressure (P) to overcome sphincter resistance. In developing that intravesical pressure, the detrusor is subject to a rule of physics (P = T/R).
According to physics (Law of Laplace), lumen pressure (P) is related to wall tension (T) and container radius (R) such that: P = f * T / R, where f = 2 for a sphere (balloon/basketball) but is unknown for the bladder (and ignored for this discussion). Because of the T = P * R relationship, a bladder containing a larger volume of urine (R) will require a higher wall tension (T) to generate needed intravesical pressure (P) for micturition. Thus, patients experiencing micturition difficulty are further compromised when their bladders are allowed to become excessively distended.
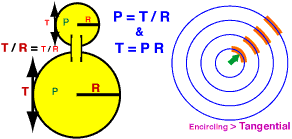 Intuitively speaking, detrusor wall tension becomes less encircling and more tangential as bladder radius expands. Compared to encircling tension, tangential tension is at a mechanical disadvantage in generating internal pressure. (It resists/generates internal pressure by shear force.) This mechanical disadvantage explains why greater wall tension is needed to generate the same lumen pressure as the bladder fills and expands.
Intuitively speaking, detrusor wall tension becomes less encircling and more tangential as bladder radius expands. Compared to encircling tension, tangential tension is at a mechanical disadvantage in generating internal pressure. (It resists/generates internal pressure by shear force.) This mechanical disadvantage explains why greater wall tension is needed to generate the same lumen pressure as the bladder fills and expands.
In terms of contraction rate and innervation dependency, smooth muscles across the body exhibit a range of values. Ureter smooth muscle is regarded as unitary (only a few pacemaker muscle cells in the renal pelvis are innervated and 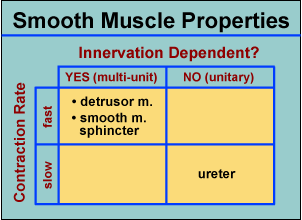 excitation spreads among individual myocytes by gap junctions). In contrast, detrusor contraction is innervation dependent while cell to cell coupling is limited. The contraction rate for bladder smooth muscle is relatively fast among smooth muscles. Thus the detrusor muscle is characterized as a multiunit, phasic type of smooth muscle.
excitation spreads among individual myocytes by gap junctions). In contrast, detrusor contraction is innervation dependent while cell to cell coupling is limited. The contraction rate for bladder smooth muscle is relatively fast among smooth muscles. Thus the detrusor muscle is characterized as a multiunit, phasic type of smooth muscle.
Detrusor myocytes have resting transmembrane potentials that range from 35 to 70 mV. The rising phase of the myocyte action potential (AP) is due to Ca++ influx through voltage gated ion channels; the AP fall is driven by K+ efflux. The Ca++ influx associated with an AP leads to additional Ca++ release from protoplasmic reticulum. The combined increased cytoplasmic [Ca++] initiates smooth muscle contraction.
Urination requires a low resistance tube able to accommodate a large volume propelled by relatively low pressure. In contrast, ejaculation requires a small urethral lumen with stiff walls. Ejaculation involves a small volume accelerated to velocity by rapid contraction of thick striated musculature surrounding the urethra.
Parasympathetic induced erection includes engorgement of the stratum spongiosum within the sub mucosa of the postprostatic urethra as well as that of the corpus spongiosum penis surrounding the penile urethra. Engorgement reduces lumen size and make the urethral wall more rigid.
Sympathetic innervation initiates ejaculation. Peristaltic waves in the ductus deferens convey spermatozoa into the lumen of the prostatic urethra. Prostatic secretion and contraction of smooth muscle surrounding prostate lobules adds prostatic secretion to the ejaculate. The smooth muscle sphincter situated in the bladder neck and proximal urethra contract to preclude ejaculation into the urinary bladder and to prevent urine contamination of the ejaculate.
 Somatic innervation completes ejaculation. Both urethralis and bulbospongiosus muscles are engaged in propelling ejaculate along the urethra.
Somatic innervation completes ejaculation. Both urethralis and bulbospongiosus muscles are engaged in propelling ejaculate along the urethra.
Based on anatomical evidence, the prostatic urethra has a passive, elastic role during ejaculation. The wall of the urethral lumen is passively stretched as sperm and prostatic fluid are added under pressure, the ejaculate being temporarily trapped by contraction of the smooth muscle sphincter cranially and the urethralis muscle caudally. Periodically, relaxation of the urethralis muscle allows ejaculate to enter the postprostatic urethra and urethralis muscle peristalsis propels an ejaculate bolus.
